In recent years, the potential to edit human genomes to treat or avoid genetic disease has been at the forefront of discussions about personalised medicine. However, using genome editing technologies is not just a question of scientific capability, but also one of ethics and governance. Should it be done? For what purposes and subject to what limitations? And, equally importantly, who should make these decisions? These questions have become more urgent – and more contentious – since 2018, when Dr He Jiankui caused international uproar by announcing the birth of twin girls whose DNA he had edited. This highlighted a familiar problem: a new technology raises complex ethical and governance issues, yet also outpaces responses to them.
In response, the World Health Organisation (WHO) established the Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, with the task to ‘advise and make recommendations on appropriate institutional, national, regional and global governance mechanisms for human genome editing’. The Advisory Committee has now released its framework for governance and a set of accompanying recommendations, marking the first serious attempt to develop international governance solutions – a welcome step towards a responsible pathway for the implementation of genome editing.
What is governance?
Governance is a broad term encompassing formal mechanisms such as law and regulations but also includes informal mechanisms such as ethical, social and professional norms that guide its development. These may be at institutional, regional, national and international levels and are often part of an evolving landscape that can be challenging to address.
The Committee’s report acknowledges that governance and regulation must be adaptive to the pace of technological and social change. It emphasises that “good governance is an iterative, ongoing process that includes mechanisms for regular revision in light of technical, practical and ethical developments and changes in societal views and values”.
The Committee’s recommendations can’t make or change international laws. However the WHO has an international moral authority, and the Organisation and its Director-General provide leadership by calling attention to both good and bad policies and practices in an effort to encourage those in positions of authority and power to support change and promote global unity.
The importance of ‘good governance’
Genome editing is often described as a ‘disruptive technology’, and therefore it is important to have good governance in place to mitigate against the potential harms. These have been well documented, and include concerns around equity of access, use for enhancement purposes and demonstrating safety and efficacy. The severity and nature of the challenges varies between somatic, germline and heritable human genome editing.
Here in the UK, we have strong and comprehensive ethical, legal and governance mechanisms that cover research and clinical applications of different types of genome editing. But many other countries do not. High profile reports in the last few years, including those by the Nuffield Council on Bioethics and National Academy of Sciences, have raised the issue of how to govern the use of these technologies effectively when they have a global reach. On one hand, there is a need for a coordinated global approach to prevent medical tourism and ‘ethics dumping’. On the other, there is a need to respect the differences between countries that are rooted in national cultural, social and religious norms. These will shape perspectives on whether and how to use genome editing and will likely lead to different regulatory approaches.
The WHO report recognises this tension. Rather than advocating for a single mechanism that is adhered to by all, it calls on all countries to incorporate certain fundamental values and principles into their policies, such as inclusiveness, equal moral worth, social justice, responsible stewardship of science, solidarity, and global health justice. An emphasis on the use of these foundational principles to promote good practice reflects the fact that the report is a framework for governance, not a complete governance framework: it aims to provide those responsible for oversight with the tools that they need rather than dictating a list of dos and don’ts.
A framework for governance
In its framework for governance, the WHO Committee considers the specific challenges that apply to different somatic and heritable genome editing applications, and identifies questions that should be considered when reviewing or creating oversight measures. It also puts forward seven scenarios which illustrate how various components of the framework might come together in practice. These range from less contentious scenarios such as clinical trials involving somatic human genome editing for Sickle Cell Disease and Huntington’s Disease to more future facing and speculative ones e.g. enhancement to improve athletic ability and heritable human genome editing for reproduction.
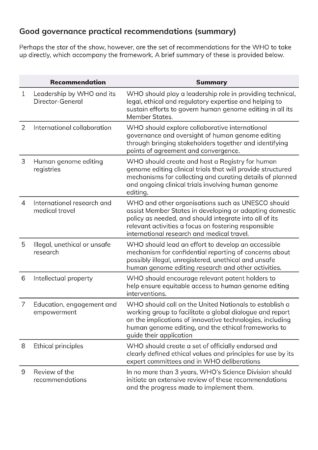
Practical recommendations
Perhaps the star of the show, however, are the set of recommendations for the WHO to take up directly, which accompany the framework. A brief summary of these is provided below.
Moving forward
The WHO has set the foundations for developing and strengthening genome editing governance frameworks, but still provides welcome flexibility and acknowledges that there is no one-size-fits-all approach to regulating genome editing on a global scale. The ball is now in the courts of national governments and professional societies to decide how they regulate genome editing in its many forms. Reassuringly, the framework does recognise the need to establish some global baseline standards, and the first of the Committee’s nine recommendations involves reiterating the 2019 statement of WHO Director-General that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing.”
Another strength of the WHO framework is its emphasis on inclusion. It advocates for helping parts of the world where there is traditionally weaker regulation of scientific and clinical research and practice, and where genome editing may not yet be invested in. This is particularly important because (a) this is where the use of unregulated human genome editing seems most likely to occur, and (b) without providing support for these jurisdictions they might miss out on the potentially considerable benefits that genome editing could confer. As highlighted in the recommendations, genome editing therapies need to be developed and made available equitably, which will require fostering innovation in low and middle income countries.
In finding governance solutions we mustn’t lose sight of the tremendous potential benefit genome editing affords – particularly, somatic cell editing. The WHO framework has revealed an entire toolbox of instruments, processes, measures and principles at our disposal from which to proactively develop ethical and legal safeguards so that genome editing – or at least some applications of it – can be allowed to progress for the benefit of all.
